Upcoming vaccine against a multi-resistant bacterium
Alpioner Therapeutics, a start-up originating from TIMC,* is developing a vaccine against Pseudomonas aeruginosa, a bacterium that is multi-resistant to antibiotics and causes serious harm to vulnerable people. Clinical trials on humans are set to begin in 2025.



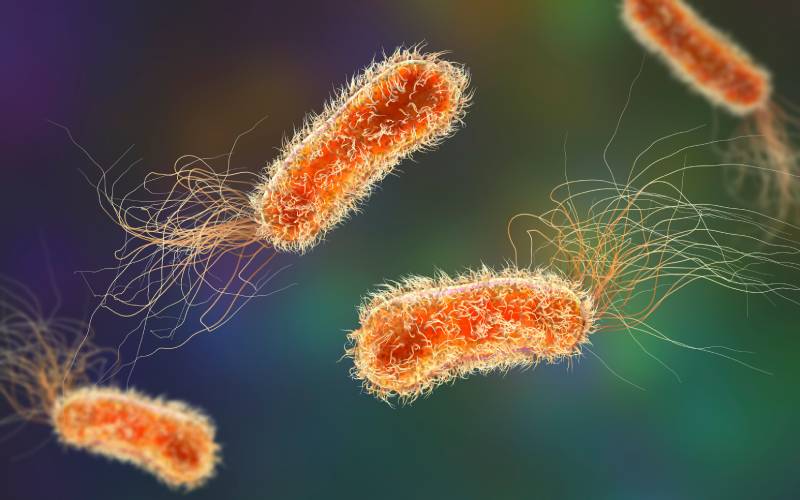
 It is the third leading cause of hospital-acquired infections, the leading cause of death in cystic fibrosis, and increases mortality in patients with chronic obstructive pulmonary disease by 50%. It caused 559,000 deaths in 2019. Pseudomonas aeruginosa is a bacterium present in 30% of the population without causing any problems, but which can become virulent in certain cases. It currently presents resistance to multiple antibiotics, and no vaccine is available on the market.
It is the third leading cause of hospital-acquired infections, the leading cause of death in cystic fibrosis, and increases mortality in patients with chronic obstructive pulmonary disease by 50%. It caused 559,000 deaths in 2019. Pseudomonas aeruginosa is a bacterium present in 30% of the population without causing any problems, but which can become virulent in certain cases. It currently presents resistance to multiple antibiotics, and no vaccine is available on the market.